PEMRYDI RTU® (pemetrexed injection) is supplied as a ready-to-use solution1
Unique HCPCS code for billing PEMRYDI RTU®: J9324.2
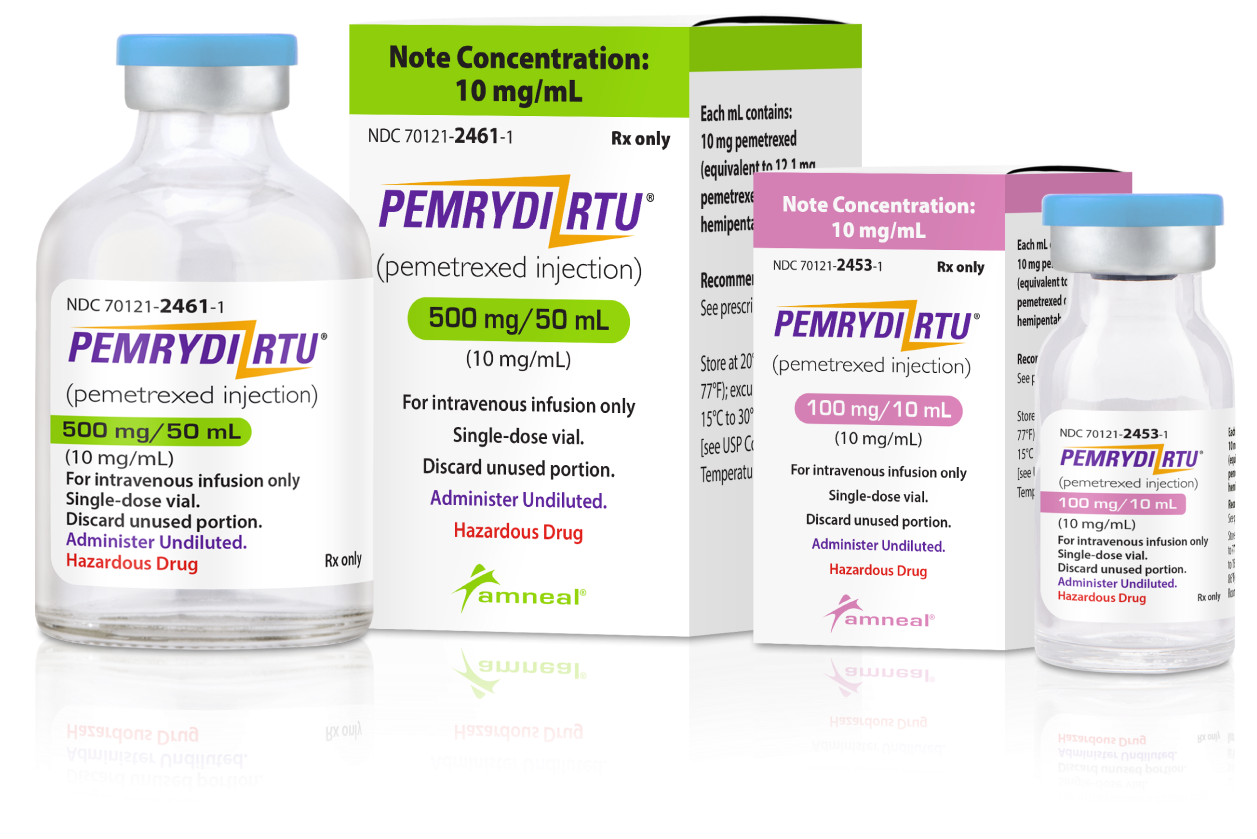
PEMRYDI RTU® is available in two strengths to minimize waste1
Unit of Sale1
Unit of Sale Quantity1
NDC1
100 mg/10 mL (10 mg/mL)
Single-dose vial
70121-2453-1
500 mg/50 mL (10 mg/mL)
Single-dose vial
70121-2461-1
Unit of Sale1
Unit of Sale Quantity1
NDC1
70121-2461-1
500 mg/50 mL (10 mg/mL)
Single-dose vial
PEMRYDI RTU® can be ordered through wholesalers and distributors.
Contact Sales@amneal.com or call (866) 525-7270 for more information.
Ready-to-use formulation makes prep more efficient
No Reconstitution
No Dilution
No Refrigeration
*500 mg/m2 as an IV infusion over 10 minutes.1
PEMRYDI RTU® may be stored in an infusion bag at controlled room temperature† for up to 24 hours prior to use. Discard the infusion bag if not used within 24 hours.1
Recommended dose of PEMRYDI RTU®1
Non-squamous non-small cell lung cancer (NSCLC)
For initial treatment of metastatic non-squamous NSCLC in combination with pembrolizumab and platinum chemotherapy1
In patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater:
The recommended dose of PEMRYDI RTU® is 500 mg/m2 as an IV infusion over 10 minutes,
administered after pembrolizumab and prior to platinum chemotherapy, on Day 1 of each 21-day cycle for 4 cycles.
Following completion of platinum-based therapy, treatment with PEMRYDI RTU® with or without pembrolizumab is administered until disease progression or unacceptable toxicity. Please refer to the full prescribing information for pembrolizumab and for carboplatin or cisplatin.
For initial treatment of locally advanced or metastatic non-squamous NSCLC in combination with cisplatin chemotherapy1
In patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater:
For maintenance treatment of locally advanced or metastatic non-squamous NSCLC as a single agent1
In patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater:
For treatment of recurrent metastatic non-squamous NSCLC as a single agent following prior chemotherapy1
In patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater:
Mesothelioma
For treatment of mesothelioma when administered with cisplatin1
In patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater:
Premedication and Concomitant Medications1
Medication
Dosage
Timing
Folic acid
400-1,000 mcg orally, once daily
Daily starting 7 days before first dose of PEMRYDI RTU®. Continue until 21 days after last dose
Vitamin B12
1 mg intramuscularly
Do not substitute oral vitamin B12 for intramuscular vitamin B12
Dexamethasone
4 mg orally, twice daily
On the day before, day of, and day after PEMRYDI RTU®
Swipe to reveal full medication information
HCPCS, Healthcare Common Procedure Coding System; IV, intravenous; NSCLC, non-small cell lung cancer.
References: 1. PEMRYDI RTU. Prescribing information. Amneal Pharmaceuticals LLC; 2025. 2. CMS. First quarter, 2024 HCPCS quarterly update. Updated March 21, 2024. https://www.cms.gov/medicare/coding-billing/healthcare-common-procedure-system/quarterly-update


